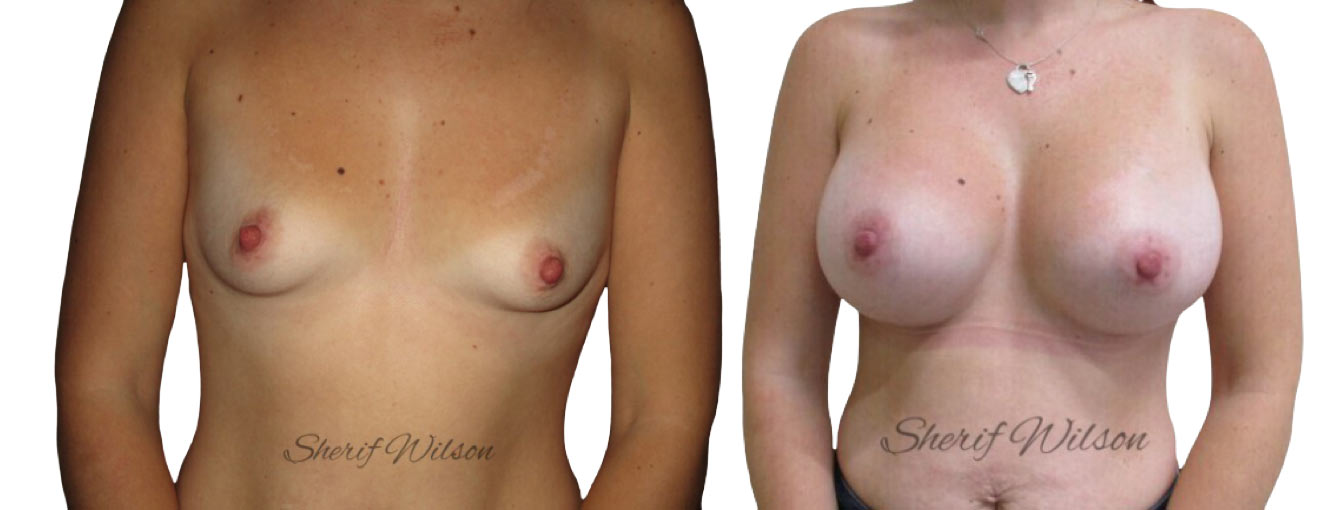
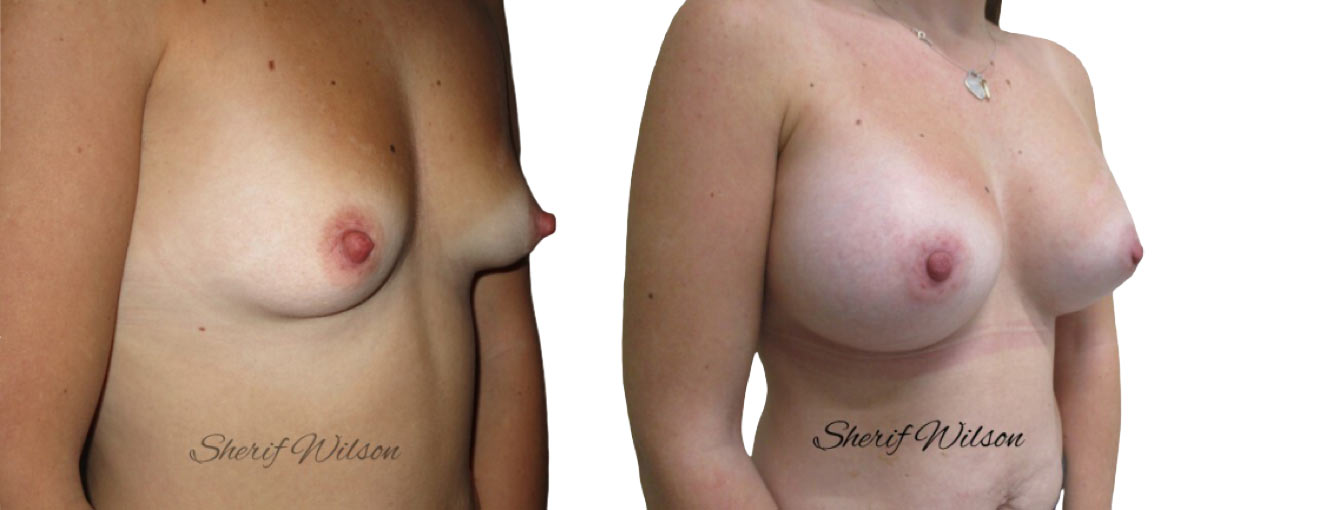
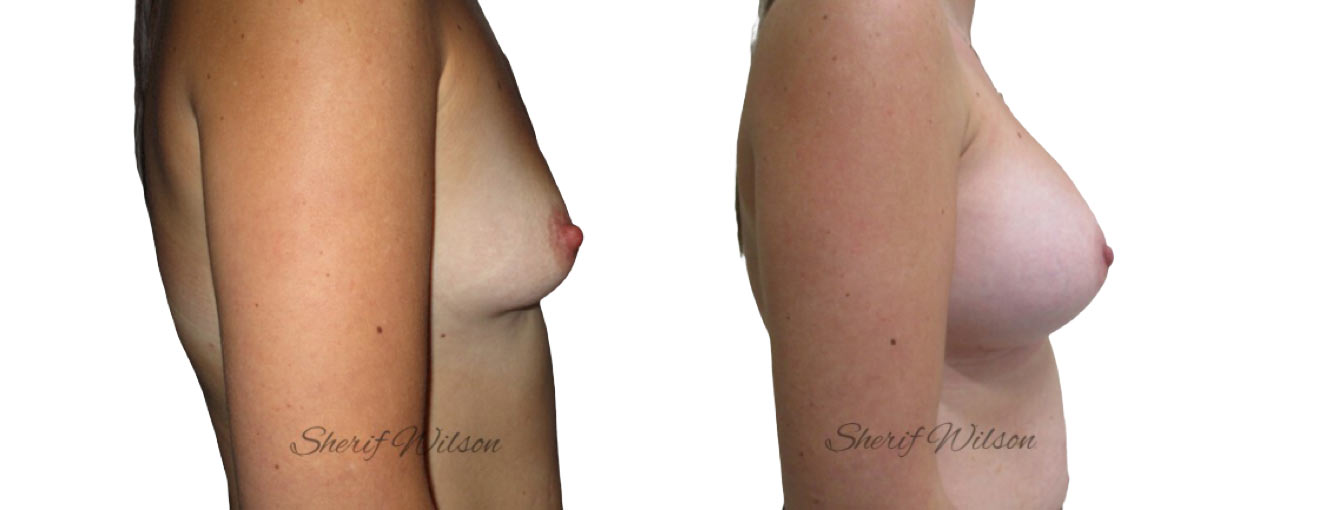
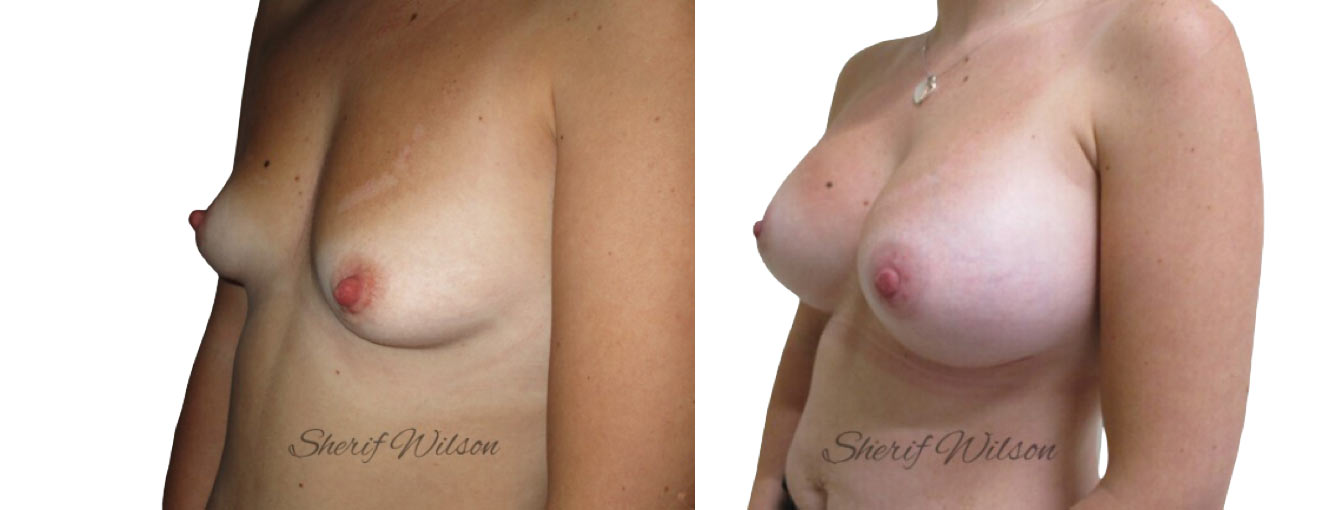
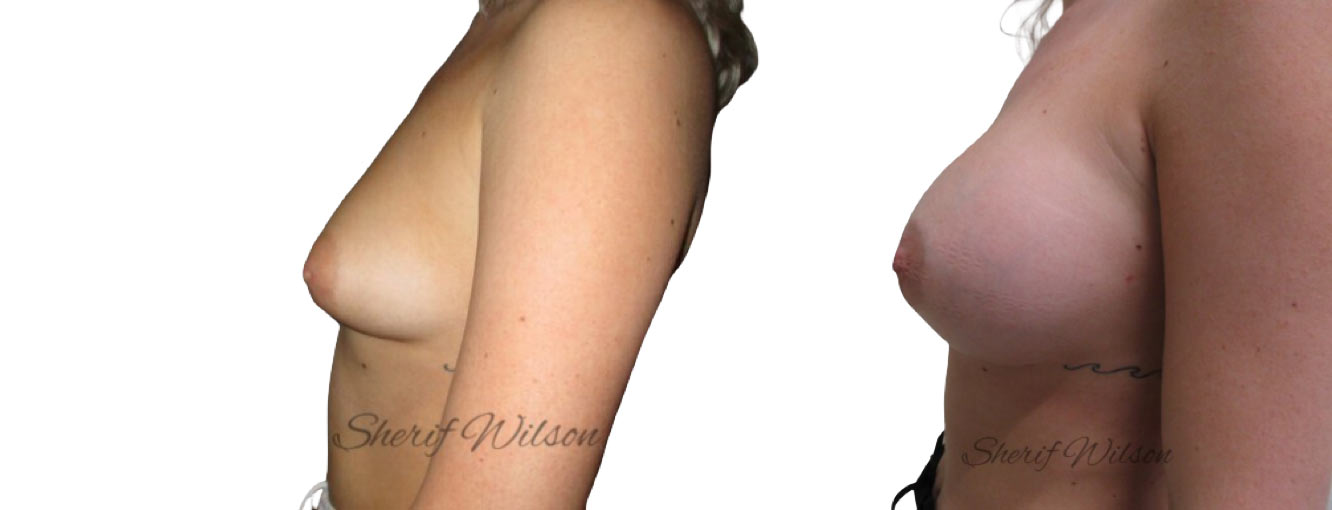
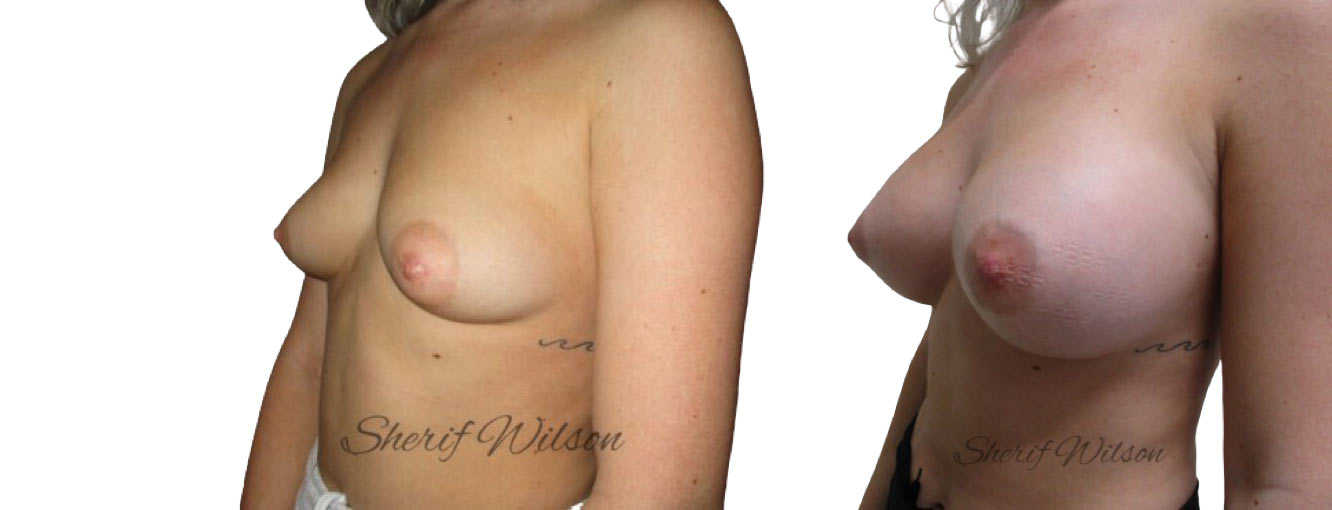
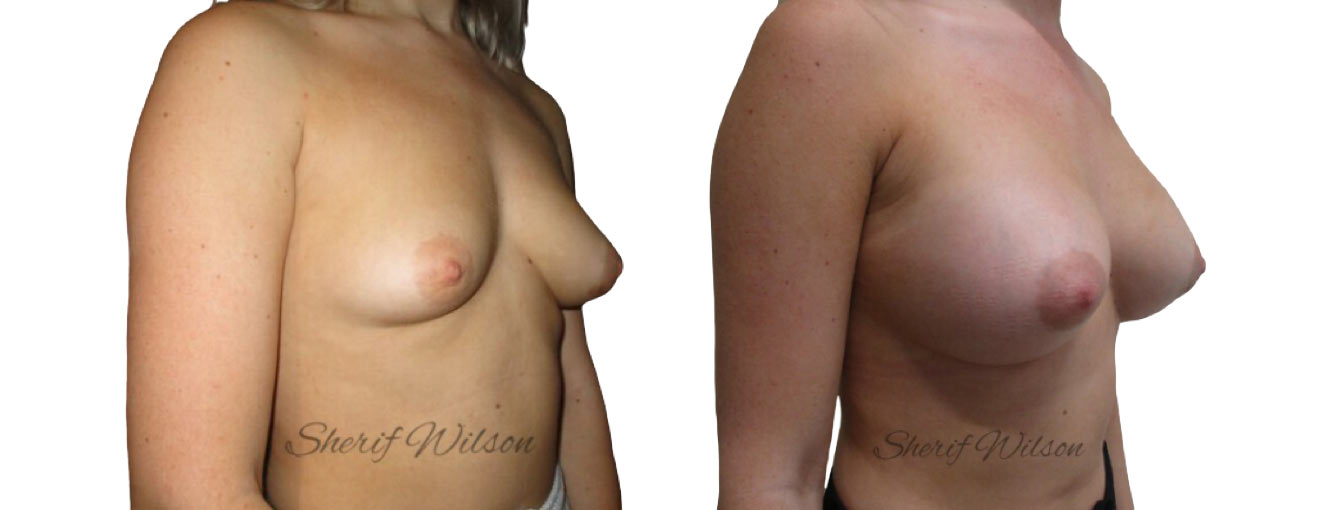
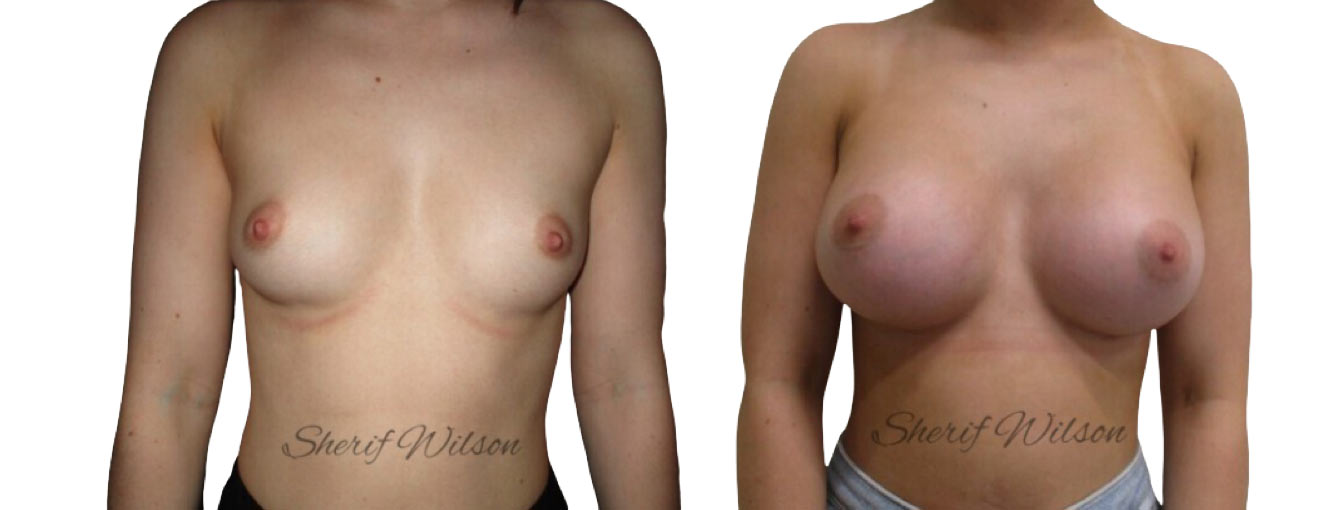
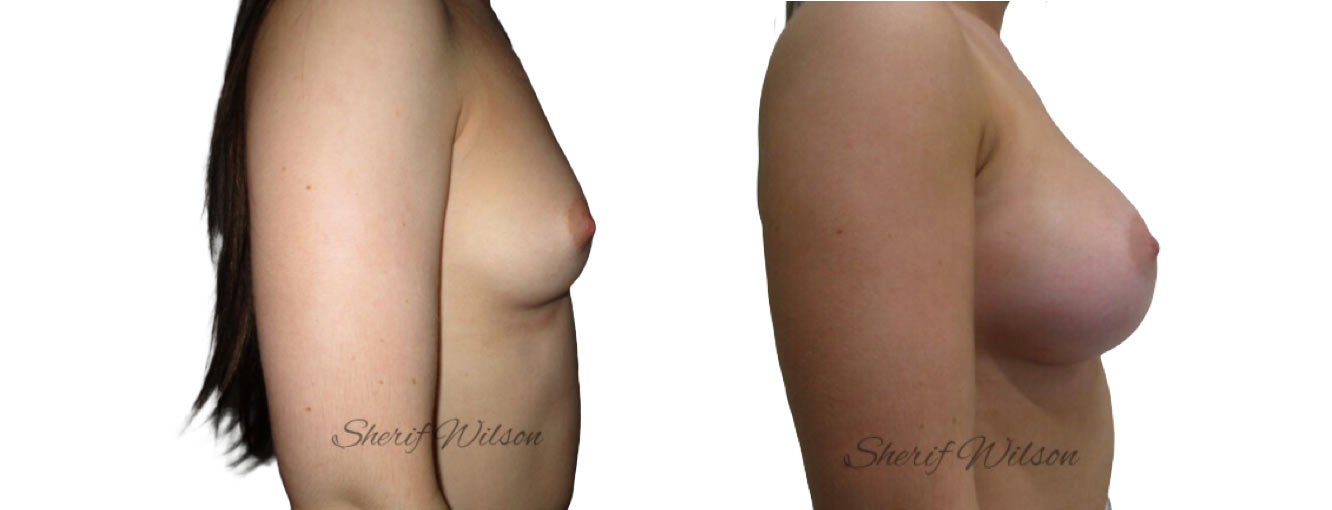
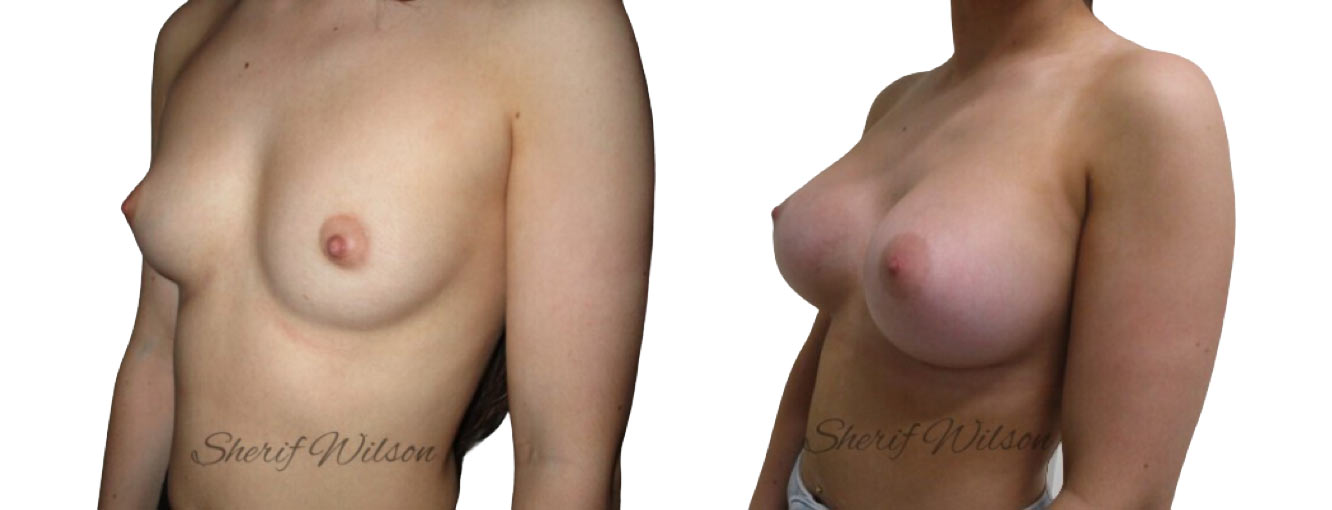
Are there risks of breast enlargement surgery?
Breast augmentation surgery is considered very safe, but as with all surgical procedures, they carry a small amount of risk.
In a low number of cases (less than 1%) breast augmentation can lead to bleeding or infection, which may result in reoperation.
Implants are made to be very tough, but in some cases, the envelope can gradually fail, and a leak can occur. This is not usually a serious event, but once detected, removal and exchange of the implant will be necessary.
Up to 10% of women (over a ten-year period), may experience hardening or encapsulation due to the formation of scar tissue around the implant.
At any point prior to your surgery, Dr Wilson will be able to talk you through the potential risks associated with breast enlargement procedures and any specific concerns you may have.
Breast Implant Associated Anaplastic Large Cell Lymphoma (BIA-ALCL)
Since 2014, a condition called anaplastic large cell lymphoma (ALCL) in association with breast implants has been identified. The risk of this is extremely small. It is not a breast cancer itself, but is associated with the scar tissue or capsule laid down by the body around a breast implant. Cases of BIA-ALCL have occurred between 2-28 years after breast implant insertion with the average time being 8 years. It is most likely to show up as a swelling around the implant causing an increase in size of the breast (a seroma). It can usually be successfully treated by an operation to remove the implant and the capsule of tissue surrounding it.
Because it is so rare, International organisations are sharing data and information about this condition. Most of the cases worldwide have occurred in women with textured breast implants, with higher numbers of BIA-ALCL seen in women with implants that have a coarser texture than those with a finer texture.
It is important to ask your surgeon what the most up-to-date recommendations are. Breast implants continue to be considered safe, with safety approval from Government organisations such as the UK MHRA and USA FDA. They continue to be used in breast reconstruction patients following treatment of cancer worldwide. For more information, please see the links at the end of this booklet.
Breast Implant Illness (BII)
Breast Implant Illness (BII) is a term used by patients who have breast implants and experience a variety of symptoms that they feel are directly connected to their silicone breast implants. BII is not a medical diagnosis and there is no proven association with breast implants. The symptoms include tiredness, “brain fog”, joint aches, immune-related symptoms, sleep disturbance, depression, hormonal issues, headaches, hair loss, chills, rash, and neurological issues.
There is currently no scientific evidence to confirm this proposed link or any diagnostic test to show that a patient suffers from such a condition. Research continues in this area to establish if all of the symptoms that patients describe can be brought together into a single diagnosis. Some patients do report that their symptoms improve if their implants are removed but this is not true for all. More guidance on BII can be found on the Gov.UK and Fda.gov websites.